INCONCRETO NEWS
Pharmaceutical industry in MOROCCO: Interview with PETER BELL from INCONCRETO
For over a year INCONCRETO has been working at Benslimane, in Morocco, as the Project Management Office (PMO) for Sensyo Pharmatech. Sensyo is a new pharmaceutical company investing in aseptic filling capabilities for vaccines in Morocco. I am delighted to share this interview with our partner Peter Bell to show you more about this interesting and impressive project. You will find Peter Bell’s profile at the end of the interview:

Carla: What is INCONCRETO doing in Morocco?
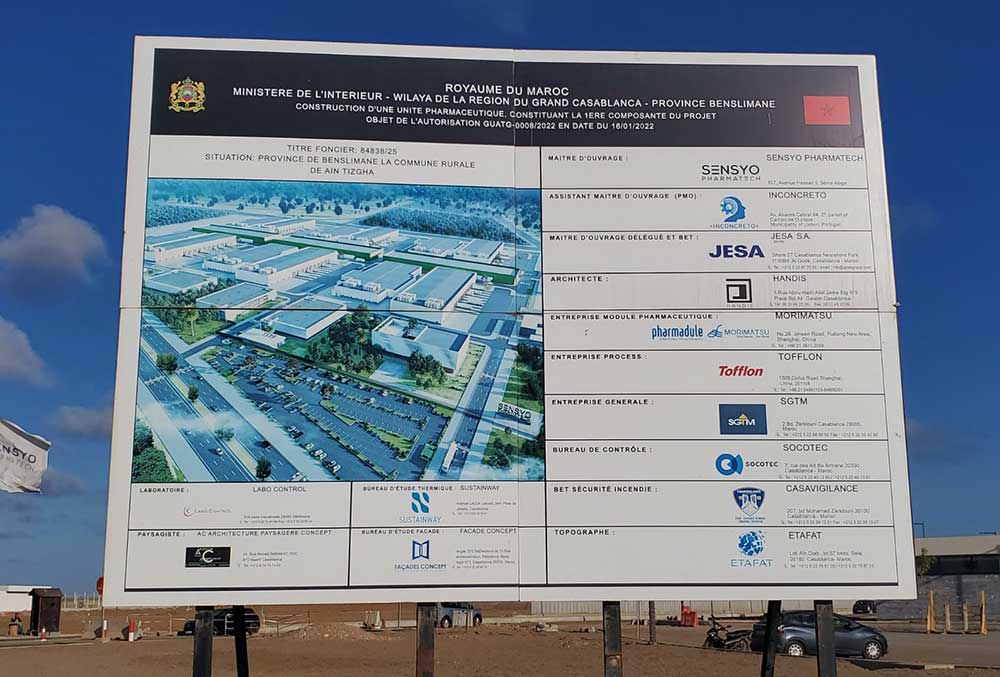
Peter Bell: To deal with the urgency to fight against Covid-19, and to allow Morocco’s self-sufficiency in vaccine supply, a decision was taken to build an aseptic filling facility for multiple vaccines, called Sensyo Pharmatech. This project has been initially designed to secure the supplies of vaccines for the Moroccan people and population, but not only, and the next step is to supply the African market as a whole. INCONCRETO has been chosen to perform the Project Management and set up the operational activities of Sensyo Pharmatech. Our role during the first phase of the studies was more about guidance and technical support for the local engineering team – to highlight the points of attention, bring potential solutions and mitigation plans to the engineers and the construction companies. Once the design was done, we gradually shifted into a more operational role, actively supporting field works, and defining a start-up strategy up to completion. This is not necessarily the prime mission of a PMO since we have managed field activities in parallel with guidance and support.
Carla: Can you tell us precisely what an aseptic filling facility is?
Peter Bell: The scope of the project today is to build the first phase, which is an aseptic filling facility with five filling lines, independent one from another, meaning that you can work simultaneously on various projects, and various products. That’s phase one. Phase two is either an expansion of additional filling lines and/or the integration of a drug substance manufacturing unit on the plot of land still available today. The facility has the ability to put in a final formulated form of vaccines, but also any other formulated drug substance, including packaging.
Today, Morocco aims to supply its own market with its vaccines and products. This is their main scope. As I said, the next step is also to supply the African continent. The future will probably dictate changes in the supply strategy or eventually bring additional supply needs. And this facility can host these new developments.
Carla: What is the possible future development of this project?
Peter Bell: The main characteristic of the facility in Benslimane is its potential for expansion. I think this is the biggest and most valuable feature of the project. Everything is ready to double the capacity if you want to expand tomorrow. You just need to build the manufacturing lines as the utilities are already there, i.e., the electrical station. The warehouse is sized for at least double the capacity of what we are doing now, if not triple. All the supporting areas are built for times two or times three of what we are doing now. The facility is set up on 11 hectares of land. The possibilities of expansion can reach 33 hectares.
The drug substance is currently not manufactured in Morocco. So, we receive the drug substance in bulk; we formulate it in its final form, and we fill the vials in an aseptic manner so that they can be readily available for the market. The Sensyo Lion 1 project will not only bring more capacity for the aseptic products in Morocco but will also enable to integrate the manufacturing of drug substances, thus having full autonomy over other therapeutic products.
Carla: In your opinion, what makes this project so unique?
Peter Bell: This project is one of a kind! In the aspect of schedule, because the initial design of this facility started in July 2021 and the completion of the project will be in December 2022, this is extremely quick. Adding to that complexity, you have two different typologies of construction: the modular coming from Asia, with equipment also coming from Asia, and traditional construction in Morocco with Moroccan teams, with worldwide suppliers (German, French, and others). So, it’s a combination of different types of construction, and many different suppliers, within a Covid context.
This means we have designed and done all the factory tests of the pharmaceutical facilities remotely by video, with absolutely no visits on-site in China to look at the installations or qualify the equipment.
Carla: But is it a good idea to do things so quickly?
Peter Bell: In a pandemic situation time is crucial, so yes, you must be fast and reactive to supply the best solution in the shortest time. Once you have your facility set up and qualified, you are available to provide high-quality products to your community.

Peter Bell’s profile:
Peter is an industrial maintenance engineer and has worked for over thirty years in the pharmaceutical industry in multiple roles: industrial maintenance, global engineering, project management, and portfolio management. He worked in some of the major pharmaceutical companies like Merck KGA in Germany, GSK vaccines in Belgium, Lonza in Switzerland, Stallergenes Greer in France, and now as a senior partner at INCONCRETO.
In the Sensyo Pharmatech project, Peter worked as a senior consultant and CQV manager. He is heading the commissioning qualification and validation of the facility: following up on the entire start-up of the facility, working on the process equipment qualification, and then supporting the validation activities, which are linked to the product.
Newsletter
© INCONCRETO. All rights reserved. Powered by AYM